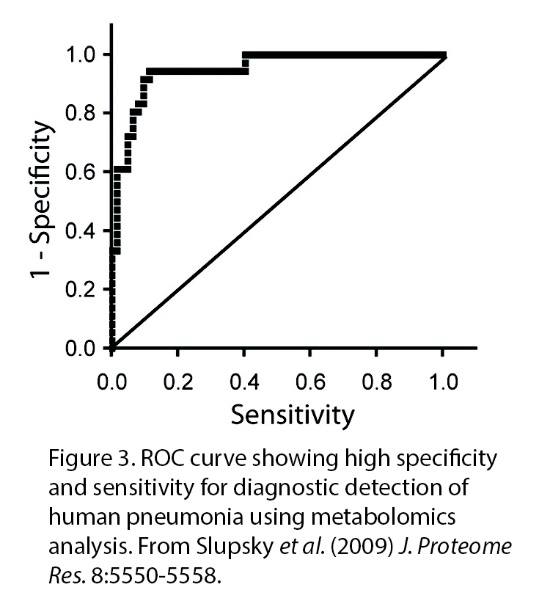
|
2nd Metabolomics - Advances &
Applications in Human Disease Conference
Venue: Boston, Massachusetts
GTCbio is proud to present the 2nd Metabolomics -
Advances & Applications in Human Disease
Conference, which will be part of The
Pan-Omics Summit and takes place May 21-22, 2015
in Boston, MA.
There is still significant human variability in
metabolite identification for targets and pathways.
These challenges affect the advancement in
metabolomics, the use of biomarkers, and its
application towards cancer, metabolic disorders, and
neurodegenerative diseases. Join us for an event that
presents new research and offers networking
opportunities with the researchers and scientists who
are working on developing clinical assays, connecting
the metabolome and the genome, and establishing common
quality standards for experimental data.
Sessions:
I. Advances in Metabolite
Markers
II. Metabolite Identification
- Targets and Pathways
III. Computational Approaches
to Assessing the Metabolome
IV. Technological Advances in
Metabolomics
Panel:
I. Clinical Applications of
Metabolomics
For more information, visit https://www.gtcbio.com/conferences/metabolomics-overview.
|